Learning Outcomes
i. Define the term "self-ionization of water".
ii. Explain the process of self-ionization of water and its consequences.
iii. Write the chemical equation representing the ionization of water.
iv. Understand the concept of hydronium and hydroxide ions.
Introduction
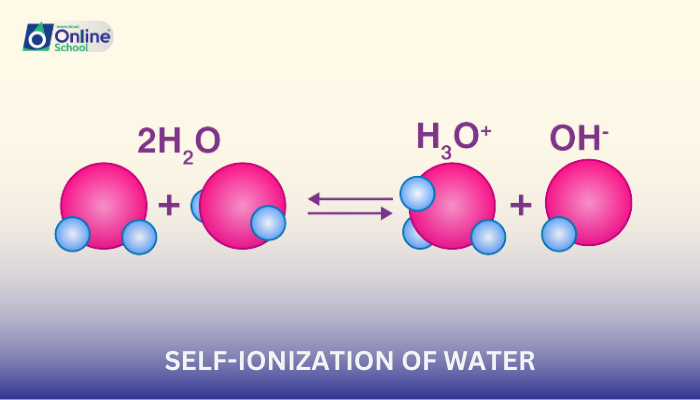
Water is often considered a neutral substance, neither acidic nor basic. However, in a seemingly counterintuitive phenomenon, water molecules can undergo a process called self-ionization, where a small fraction of water molecules dissociate into hydrogen ions (H+) and hydroxide ions (OH–).
i. Self-Ionization: A Dynamic Equilibrium
Self-ionization of water is a dynamic equilibrium process, meaning that the forward and reverse reactions occur simultaneously. The equation representing this equilibrium is:
H₂O (l) ⇌ H+ (aq) + OH– (aq)
In this equation, the double arrow (⇌) indicates that the reaction can proceed in either direction.
ii. Equilibrium Constant of Water (Kw)
The equilibrium constant of water (Kw) is a measure of the extent to which water dissociates into ions. At 25°C, Kw is approximately 1.0 x 10-14. This means that in a liter of pure water, only about 1 in 100 million water molecules is dissociated at any given time.
iii. Consequences of Self-Ionization
The self-ionization of water has two important consequences:
Water as an Amphoteric Substance: Water can act as both an acid and a base. When it donates a proton to a stronger base, it acts as an acid. When it accepts a proton from a stronger acid, it acts as a base.
Electrical Conductivity of Water: The presence of H+ and OH– ions allows water to conduct electricity, albeit to a much lesser extent than strong electrolytes like ionic compounds.
iv. Hydronium and Hydroxide Ions
In aqueous solutions, H+ ions readily combine with water molecules to form hydronium ions (H3O+). This is represented by the equation:
H+ (aq) + H2O (l) ⇌ H3O+ (aq)
OH– ions, on the other hand, remain as such in aqueous solutions.
The self-ionization of water is a fundamental concept in chemistry that has far-reaching implications. It is essential for understanding the pH scale, acid-base chemistry, and the behavior of aqueous solutions. By comprehending this process, we gain insights into the remarkable properties of water and its role in various chemical and biological systems.